 Home
Home
 Back
Back
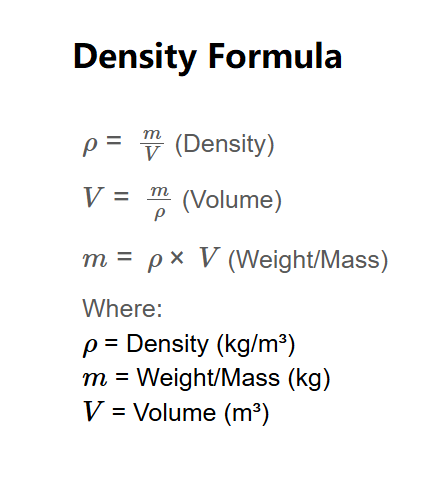
Density (\( \rho \)) is the mass per unit volume of a material, typically measured in kg/m³. Volume (\( V \)) is the space occupied by the material, typically in m³. Weight (or mass, \( m \)) is the quantity of matter, typically in kg. These three quantities are related by the density formula, which allows you to calculate one if the other two are known.
For example, a material with a weight of 100 kg and a volume of 0.1 m³ has a density of 1000 kg/m³.
Formulas:
\( \rho = \frac{m}{V} \) (Density)
\( V = \frac{m}{\rho} \) (Volume)
\( m = \rho \times V \) (Weight/Mass)
Where:
Example Calculation:
Use the form above to select what to calculate (default is Density), and the corresponding input field will be hidden. Input the known values with their respective units to get the result in multiple units.
| Unit | Conversion to kg/m³ |
|---|---|
| kilogram/cubic meter (SI Unit) | 1 |
| kilogram/cubic centimeter | 1,000,000 |
| gram/cubic meter [g/m³] | 0.001 |
| gram/cubic centimeter | 1000 |
| kilogram/liter [kg/L] | 1000 |
| gram/liter [g/L] | 1 |
| pound/cubic inch [lb/in³] | 27,680 |
| pound/cubic foot [lb/ft³] | 16.02 |
| pound/cubic yard [lb/yd³] | 0.5933 |
| pound/gallon (US) | 119.83 |
| pound/gallon (UK) | 99.78 |
| ounce/cubic inch [oz/in³] | 1,730 |
| ounce/cubic foot [oz/ft³] | 1.001 |
| ounce/gallon (US) | 7.489 |
| ounce/gallon (UK) | 6.236 |
| ton (short)/cubic yard | 1,186.6 |
| ton (long)/cubic yard | 1,328.9 |
| psi/1000 feet | 2.3067 |
| Material | Density in kg/m³ |
|---|---|
| Earth's atmosphere at sea level | 1.2 |
| Water at standard temperature and pressure | 1,000 |
| The Earth | 5,515.3 |
| Iron | 7,874 |
| Copper | 8,950 |
| Tungsten | 19,250 |
| Gold | 19,300 |
| Platinum | 21,450 |
| Atomic nuclei | 2.3×10¹⁷ |
| Black hole | above 1×10¹⁸ |
What is density?
The density of a material is the amount of mass it has per unit volume. A material with a higher density will weigh more than another material with a lower density if they occupy the same volume.
How do I find density?
How can I find volume with density and mass?
What is the formula for density?
The formula for density is the mass of an object divided by its volume. In equation form, that's \( d = \frac{m}{v} \), where \( d \) is the density, \( m \) is the mass, and \( v \) is the volume of the object. The standard units are kg/m³.
How do I find density of a liquid?
Which planet has the lowest density?
Of the eight planets in the Solar System, Saturn has the lowest density at 687 kg/m³. This is much less than the density of water at 1,000 kg/m³. So, if you could put Saturn on a body of water, it would float!
Which element has the greatest density at standard temperature and pressure?
Osmium is the densest element on the periodic table that occurs naturally, with a density of 22,590 kg/m³. It is combined with other metals to make the tips of fountain pen nibs, electrical contacts, and in other high-wear applications.
How do I measure the density of an irregular object?
How do I calculate the density of the Earth?
How can I find mass with density and volume?