 Home
Home
 Back
Back
Specific Gravity (SG), also known as relative density, is a dimensionless unit defined as the ratio of the density of a substance to the density of water at a specified temperature. Commonly, the reference temperature is 4°C (39°F), where water has its highest density of approximately 1000 kg/m³ (1.940 slugs/ft³). Specific gravity is used to compare the density of a substance to that of water, making it useful in fields like chemistry, engineering, and geology.
For example, a substance with a density of 1500 kg/m³ has a specific gravity of 1.5000.
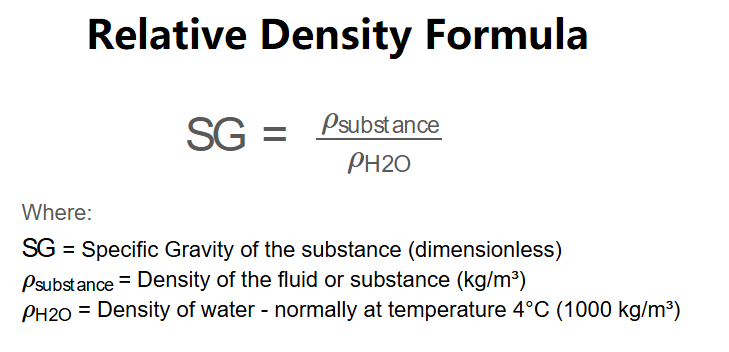
Conversion Formula: The specific gravity is calculated as:
\(\text{SG} = \frac{\rho_{\text{substance}}}{\rho_{\text{H2O}}}\)
Where:
Example Calculation: For a density of 1500 kg/m³:
In the Imperial system, using slugs/ft³ (1 kg/m³ ≈ 0.00194032 slugs/ft³, and water at 4°C ≈ 1.940 slugs/ft³), the SG remains the same numerically due to the dimensionless nature.
Use the form above to input the density in kg/m³, and the calculator will compute the specific gravity in both SI and Imperial systems.
The calculator accepts density in kilograms per cubic meter (kg/m³) and computes specific gravity, which is dimensionless. The reference density of water is:
Other common density conversions include:
Specific gravity is the same numerically in both SI and Imperial systems when using consistent reference densities.
Some standard specific gravities include:
Use this calculator to determine the specific gravity for these substances or your own measurements.
How do you calculate specific gravity?
To calculate specific gravity:
What is the specific gravity of water at 4°C?
The specific gravity is \(1000 / 1000 = 1.0000\).
Does specific gravity differ between SI and Imperial systems?
No, specific gravity is dimensionless and has the same numerical value in both systems when using consistent reference densities (e.g., water at 4°C).