 Home
Home
 Back
Back
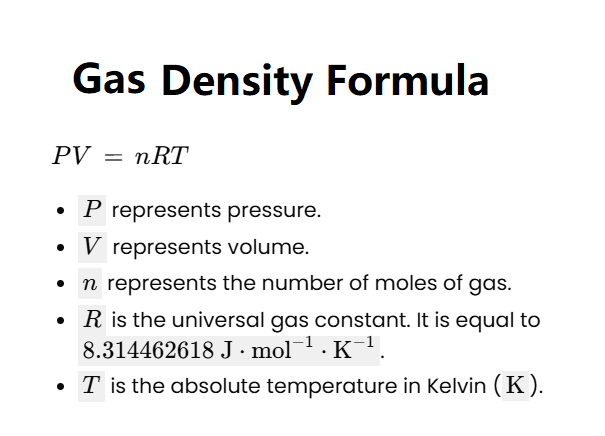
Gas density is the mass of a gas per unit volume, typically measured in kg/m³. It depends on the pressure, temperature, and molecular mass of the gas. According to the ideal gas law, gas density can be derived as \(\rho = \frac{P \cdot M}{R \cdot T}\), where \(P\) is pressure, \(M\) is the molecular mass, \(R\) is the universal gas constant, and \(T\) is the temperature in Kelvin. Higher pressure increases the density by compressing more gas molecules into a given volume, while higher temperature decreases density by causing the gas to expand. The molecular mass also plays a role—gases with higher molecular masses (e.g., CO₂ at 44 g/mol) are denser than those with lower molecular masses (e.g., H₂ at 2 g/mol) under the same conditions.
For example, at standard temperature and pressure (STP, 0 °C and 1 atm), the density of air (average molecular mass ~29 g/mol) is about 1.293 kg/m³. This calculator allows you to compute the density of any ideal gas by inputting its pressure, temperature, and molecular mass.
Gas Density Calculation: The gas density is calculated using the ideal gas law in the form:
\(\rho = \frac{P \cdot M}{R \cdot T}\)
Where:
Example Calculation: Let’s calculate the density of a gas with a pressure of 2000 Pa, temperature of 2000 K, and molecular mass of 555 g/mol (as shown in the image):
Use the form above to input your values with the desired units, and the calculator will convert them internally to provide the gas density in multiple units.
The SI unit for density is kilogram per cubic meter (kg/m³). Other convenient units include:
Imperial units for density include:
The calculator displays gas density in multiple units for convenience, allowing you to choose the unit that best suits your needs.
Gas density varies with temperature and pressure, so standard reference conditions are defined for consistency. Common standards include:
For example, the density of air (molecular mass ~29 g/mol) at STP is:
\(\rho = \frac{101325 \cdot (29 / 1000)}{8.314462618 \cdot 273.15} \approx 1.293 \, \text{kg/m³}\)
Choose the appropriate standard conditions based on your application to calculate the standard gas density using this calculator.
The molecular mass (\(M\)) of a gas is the mass of one mole of its molecules, typically measured in g/mol or kg/mol. It’s calculated by summing the atomic masses of all atoms in the molecule. For example:
The molecular mass affects the density of the gas—higher molecular masses result in higher densities under the same pressure and temperature conditions.
How do you calculate gas density?
To calculate the density of a gas:
What is the density of air at STP?
The density of air (average molecular mass ~29 g/mol) at STP (0 °C, 1 atm) is approximately 1.293 kg/m³, as calculated above.